Mass Number: Understanding the Backbone of Atomic Identity
Learn about mass number in chemistry—understand atomic structure, isotopes, and the importance of protons and neutrons in determining an atom’s identity.
In chemistry, the concept of the mass number is crucial for understanding the unique identity of each element’s atoms. Simply put, the mass number is the sum of protons and neutrons present in an atom's nucleus. This single number holds the key to identifying different isotopes of an element, understanding nuclear reactions, and even predicting atomic stability. Let's dive into the details of this fundamental concept and discover why it's so important in chemistry.
What is Mass Number?
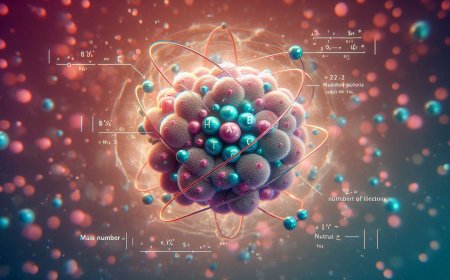
The mass number, often represented by the symbol "A," is defined as the total number of protons and neutrons in the nucleus of an atom. Protons and neutrons are the two major types of subatomic particles within the nucleus, with protons carrying a positive charge and neutrons being neutral. Since electrons, which carry a negative charge, are much lighter than protons and neutrons, they are not included in the mass number calculation.
Mathematically, it is represented as:
Mass Number (A) = Number of Protons + Number of Neutrons
For example, the mass number of a carbon atom with 6 protons and 6 neutrons would be 12.
Why is Mass Number Important?
- Isotope Identification: The mass number is essential for distinguishing isotopes—atoms of the same element with different numbers of neutrons. For instance, carbon-12 and carbon-14 both have 6 protons (which makes them carbon), but carbon-12 has 6 neutrons, while carbon-14 has 8. The difference in mass numbers (12 and 14) differentiates these isotopes and gives each unique properties, especially in areas like radioactive dating.
- Determining Atomic Stability: The ratio of protons to neutrons affects an atom’s stability. Atoms with too many or too few neutrons compared to protons may become unstable, leading to radioactive decay. Thus, the mass number indirectly influences whether an atom is stable or radioactive.
- Chemical Reactions and Nuclear Reactions: While the mass number is not directly involved in chemical reactions (which primarily involve electrons), it becomes significant in nuclear reactions. In nuclear fission and fusion, for example, the mass numbers of the reactants and products play a crucial role in understanding and calculating the energy changes that occur.
- Practical Applications: Understanding mass numbers allows chemists to identify elements and their isotopes in fields like medicine, energy production, and environmental science. Isotopes of elements are used in a variety of applications, from medical imaging to power generation in nuclear reactors.
Calculating Mass Number
Calculating the mass number of an atom is straightforward. If you know the number of protons (atomic number) and the number of neutrons, simply add them together. For example:
- For oxygen-16, with 8 protons and 8 neutrons:
Mass Number (A) = 8 + 8 = 16
- For uranium-238, with 92 protons and 146 neutrons:
Mass Number (A) = 92 + 146 = 238
Mass Number vs. Atomic Mass
A common point of confusion is the distinction between mass number and atomic mass. While the mass number is an integer representing the total count of protons and neutrons in a single atom, atomic mass is the weighted average mass of all isotopes of an element found in nature, typically expressed in atomic mass units (amu).
Conclusion
The mass number may seem like a simple counting exercise, but it serves as a gateway to understanding a variety of chemical phenomena, from the identification of isotopes to the study of nuclear processes. It helps scientists classify atoms, predict stability, and even unlock powerful applications that benefit society. The next time you look at the periodic table, remember: the mass number is at the heart of an element’s atomic structure and identity.
What's Your Reaction?